Deposition Date
1998-02-12
Release Date
1998-05-27
Last Version Date
2021-11-03
Entry Detail
PDB ID:
1A5A
Keywords:
Title:
CRYO-CRYSTALLOGRAPHY OF A TRUE SUBSTRATE, INDOLE-3-GLYCEROL PHOSPHATE, BOUND TO A MUTANT (ALPHAD60N) TRYPTOPHAN SYNTHASE ALPHA2BETA2 COMPLEX REVEALS THE CORRECT ORIENTATION OF ACTIVE SITE ALPHA GLU 49
Biological Source:
Source Organism:
Salmonella typhimurium (Taxon ID: 602)
Host Organism:
Method Details:
Experimental Method:
Resolution:
1.90 Å
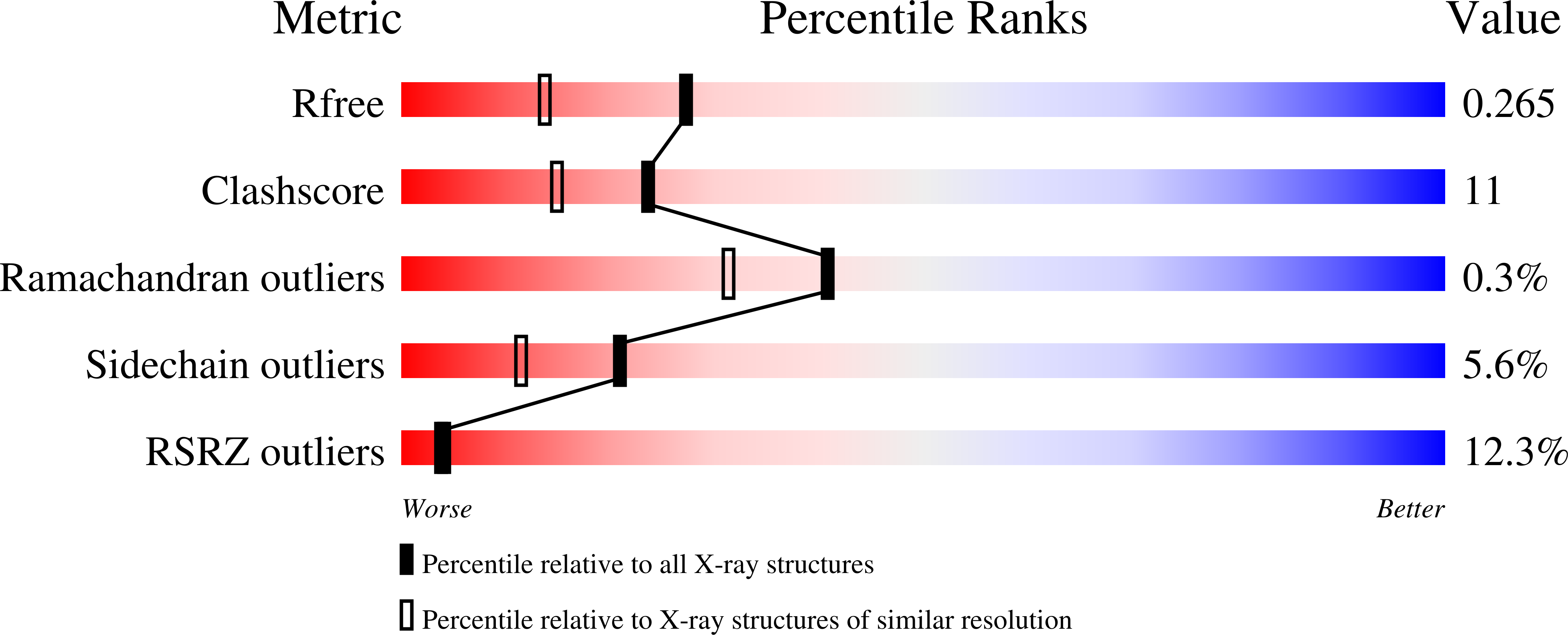
R-Value Free:
0.29
R-Value Work:
0.23
R-Value Observed:
0.23
Space Group:
C 1 2 1