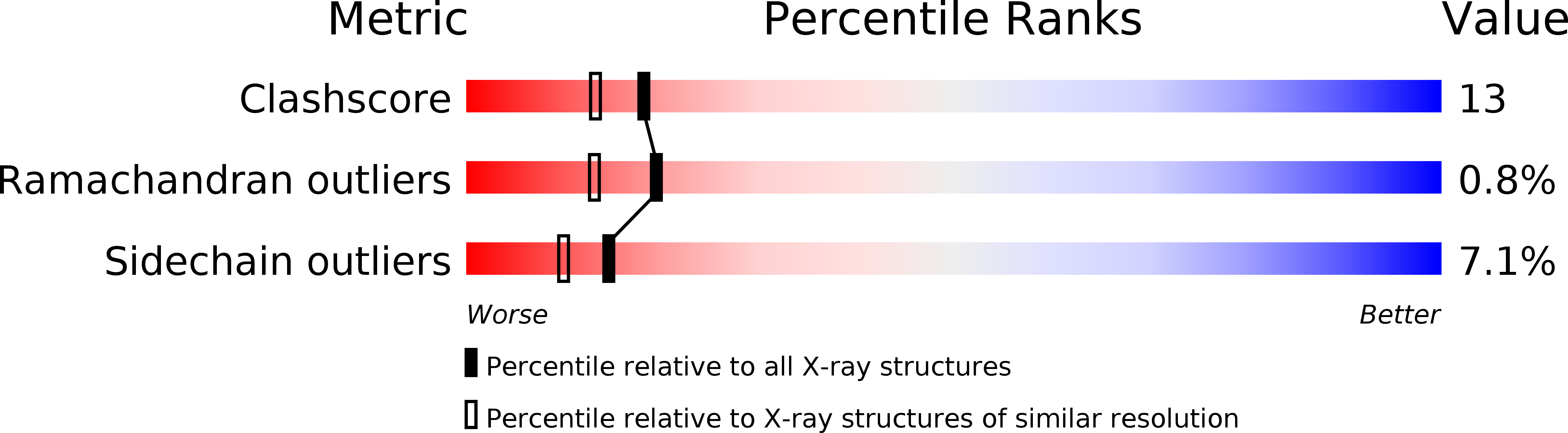
Deposition Date
1998-01-11
Release Date
1998-04-29
Last Version Date
2023-08-02
Entry Detail
PDB ID:
1A2U
Keywords:
Title:
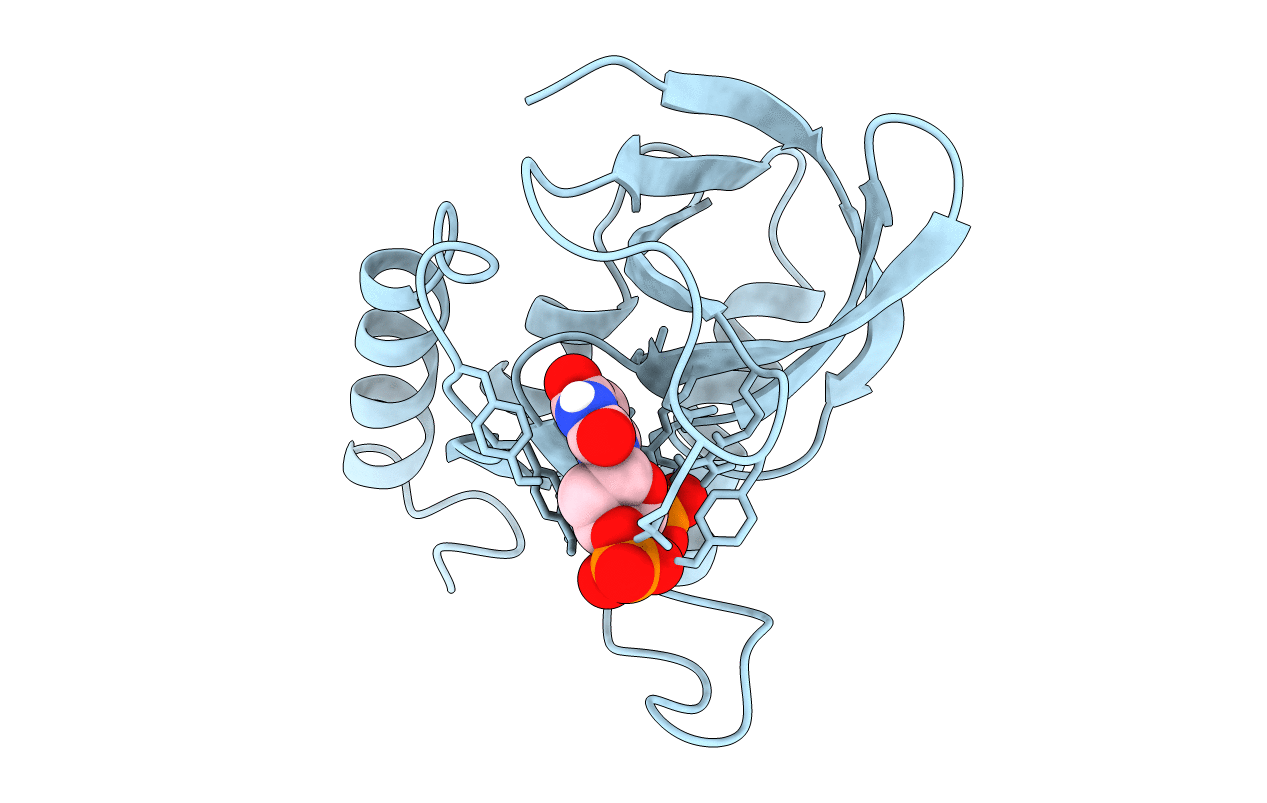
STAPHYLOCOCCAL NUCLEASE, V23C VARIANT, COMPLEX WITH 1-N-BUTANE THIOL AND 3',5'-THYMIDINE DIPHOSPHATE
Biological Source:
Source Organism(s):
Staphylococcus aureus (Taxon ID: 1280)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Work:
0.17
R-Value Observed:
0.17
Space Group:
P 41