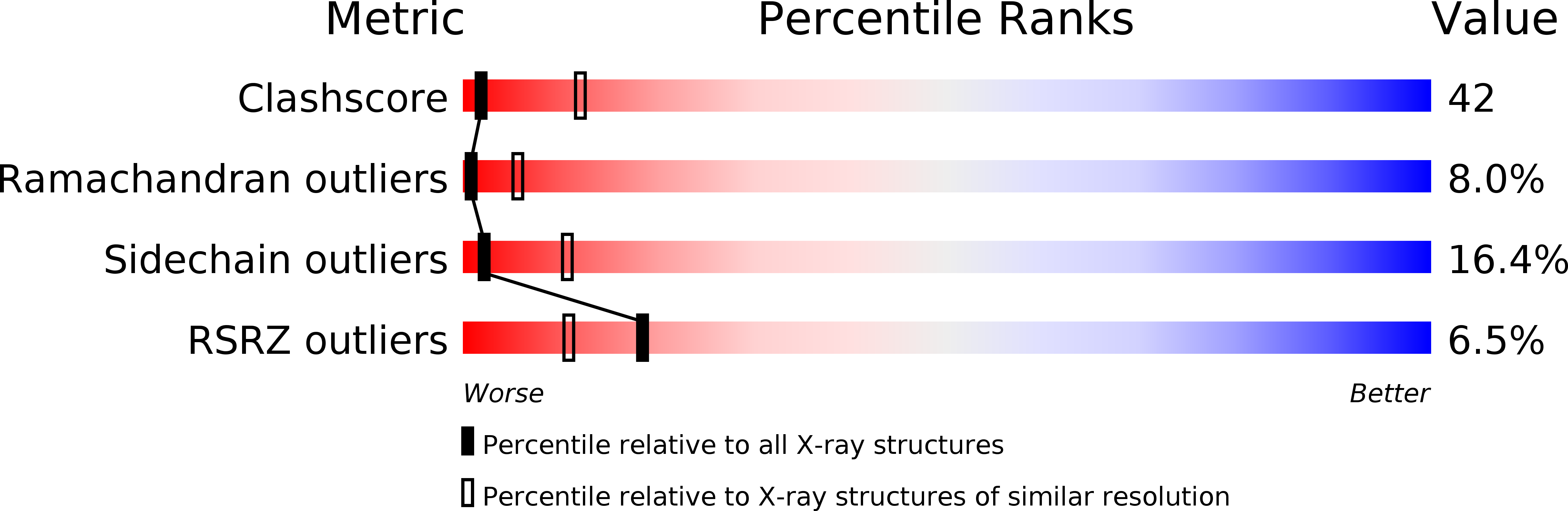Deposition Date
1997-11-30
Release Date
1998-06-17
Last Version Date
2024-11-13
Entry Detail
PDB ID:
1A0H
Keywords:
Title:
THE X-RAY CRYSTAL STRUCTURE OF PPACK-MEIZOTHROMBIN DESF1: KRINGLE/THROMBIN AND CARBOHYDRATE/KRINGLE/THROMBIN INTERACTIONS AND LOCATION OF THE LINKER CHAIN
Biological Source:
Source Organism(s):
Bos taurus (Taxon ID: 9913)
Method Details:
Experimental Method:
Resolution:
3.20 Å
R-Value Free:
0.24
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
P 41 21 2