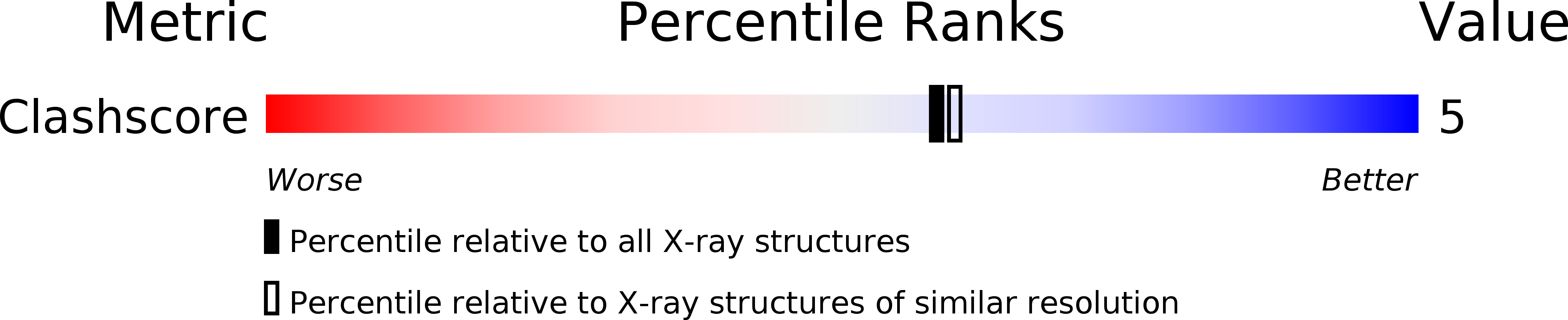
Deposition Date
1994-04-08
Release Date
1994-08-31
Last Version Date
2024-02-07
Entry Detail
PDB ID:
168D
Keywords:
Title:
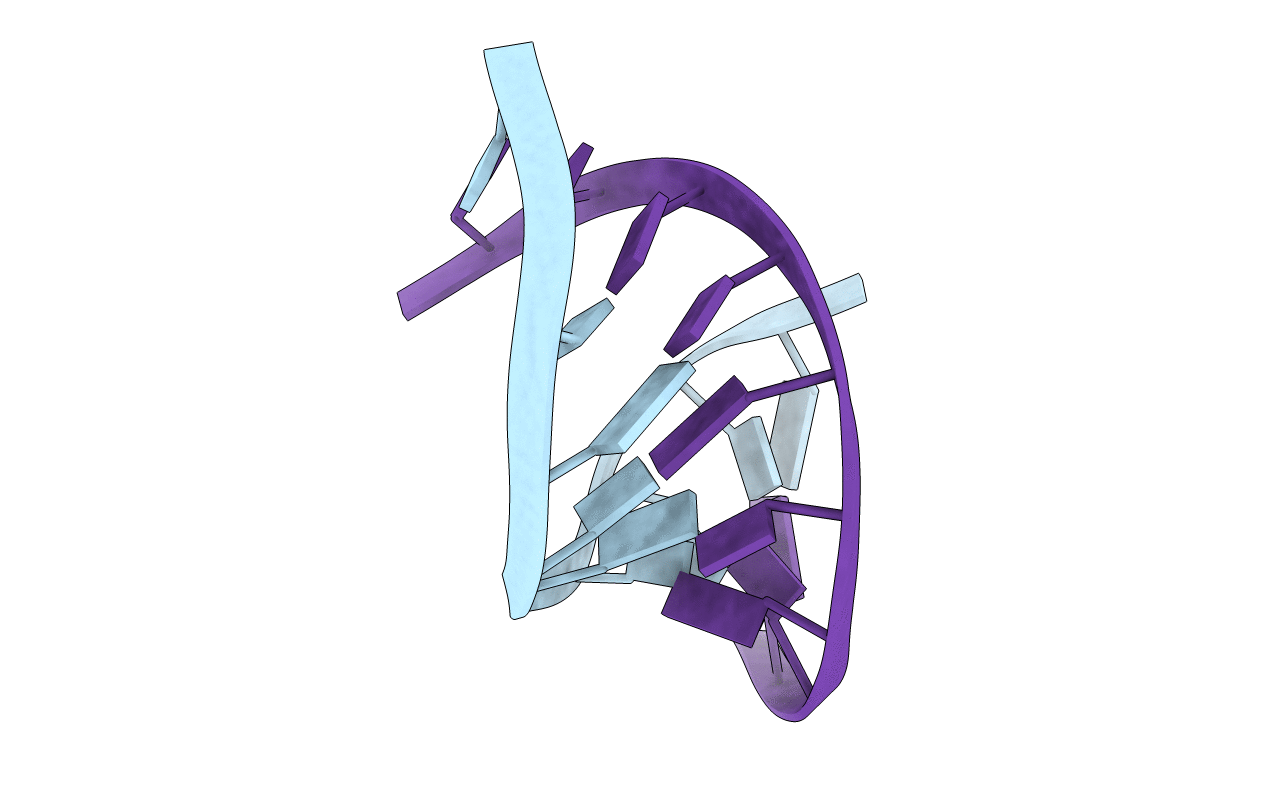
STABILIZING EFFECTS OF THE RNA 2'-SUBSTITUENT: CRYSTAL STRUCTURE OF AN OLIGODEOXYNUCLEOTIDE DUPLEX CONTAINING 2'-O-METHYLATED ADENOSINES
Method Details:
Experimental Method:
Resolution:
2.00 Å
R-Value Observed:
0.19
Space Group:
P 21 21 21