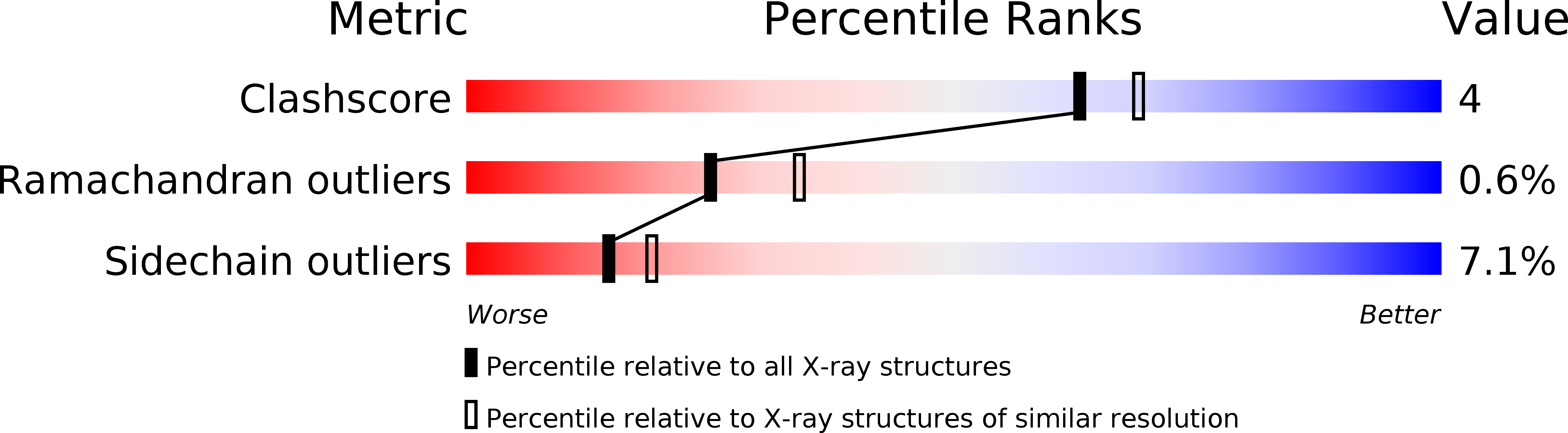
Deposition Date
1998-08-10
Release Date
1999-02-09
Last Version Date
2023-08-09
Entry Detail
PDB ID:
10MH
Keywords:
Title:
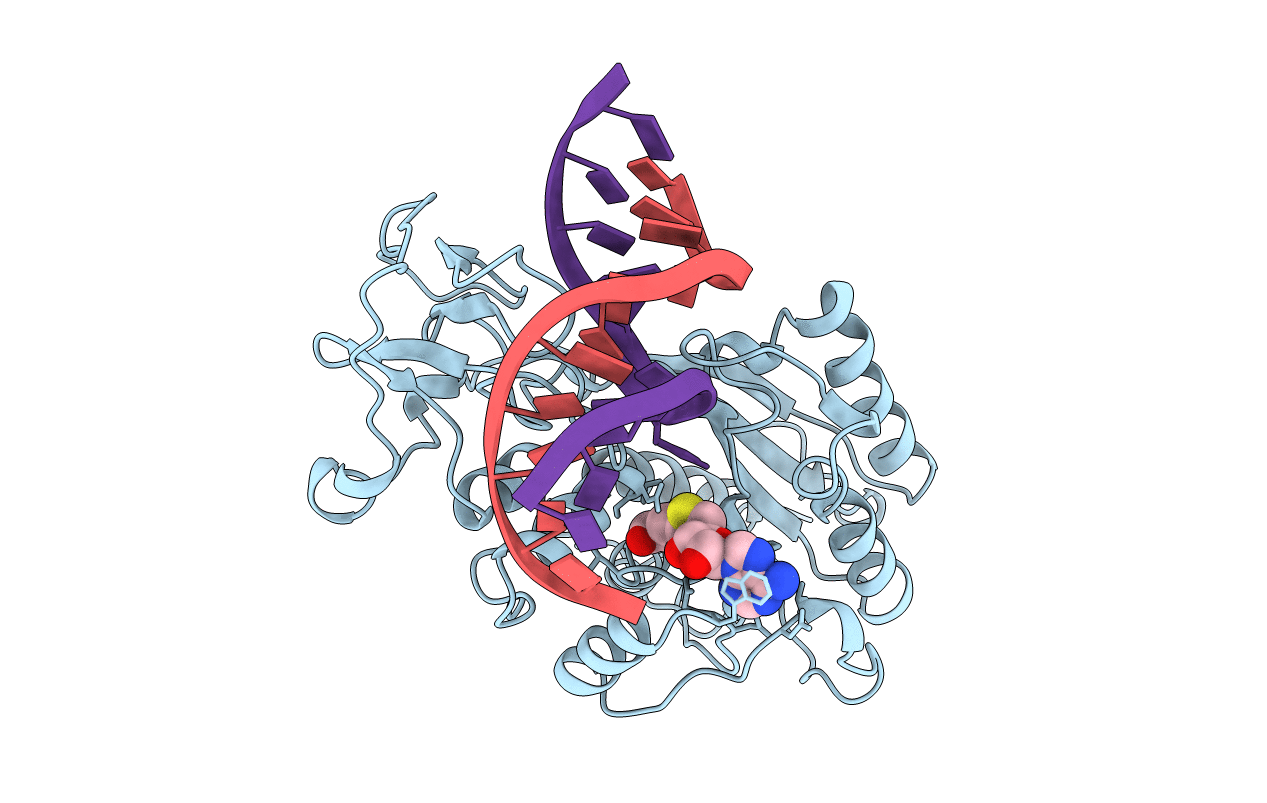
TERNARY STRUCTURE OF HHAI METHYLTRANSFERASE WITH ADOHCY AND HEMIMETHYLATED DNA CONTAINING 5,6-DIHYDRO-5-AZACYTOSINE AT THE TARGET
Biological Source:
Source Organism(s):
Haemophilus haemolyticus (Taxon ID: 726)
Expression System(s):
Method Details:
Experimental Method:
Resolution:
2.55 Å
R-Value Work:
0.20
R-Value Observed:
0.20
Space Group:
H 3 2