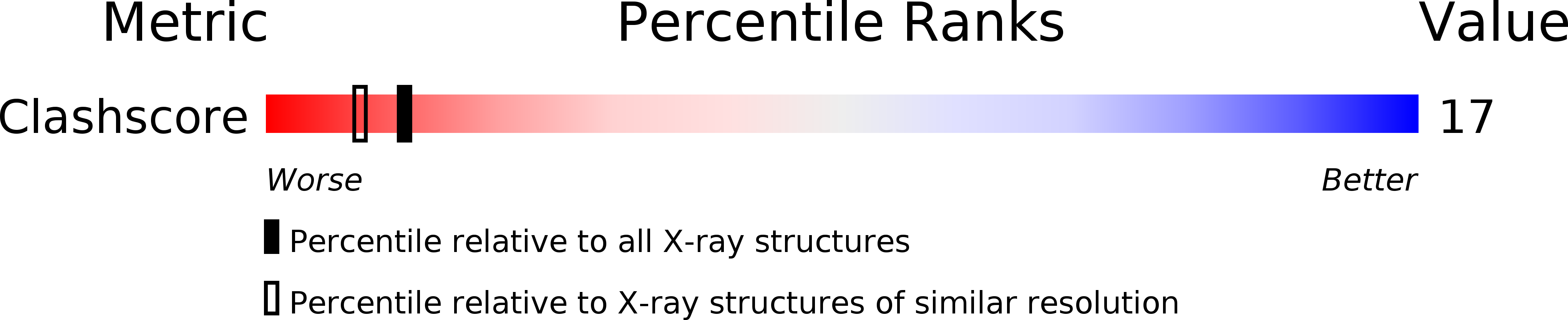Deposition Date
1994-12-14
Release Date
1995-02-27
Last Version Date
2023-11-22
Entry Detail
PDB ID:
101D
Keywords:
Title:
REFINEMENT OF NETROPSIN BOUND TO DNA: BIAS AND FEEDBACK IN ELECTRON DENSITY MAP INTERPRETATION
Method Details:
Experimental Method:
Resolution:
2.25 Å
R-Value Free:
0.25
R-Value Observed:
0.16
Space Group:
P 21 21 21