Search Count: 16
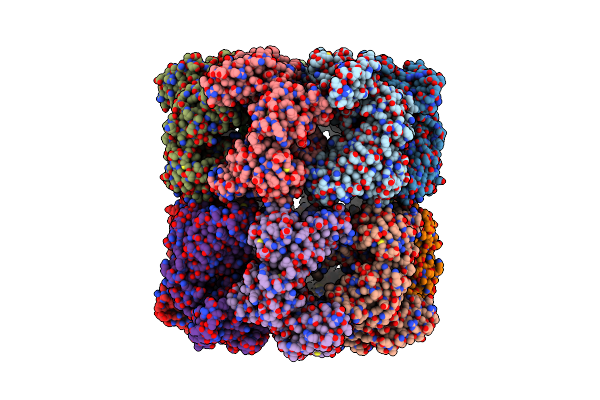 |
Cryo-Em Structure Of Groel Bound To Unfolded Substrate (Ugt1A) At 2.8 Ang. Resolution (Consensus Refinement)
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
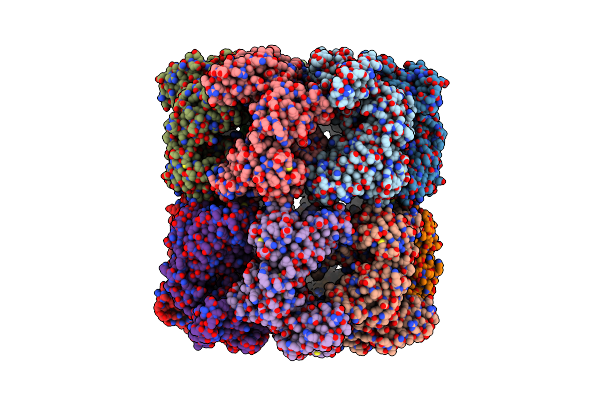 |
Cryo-Em Structure Of Double Occupied Ring (Dor) Of Groel-Ugt1A Complex At 2.7 Ang. Resolution
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
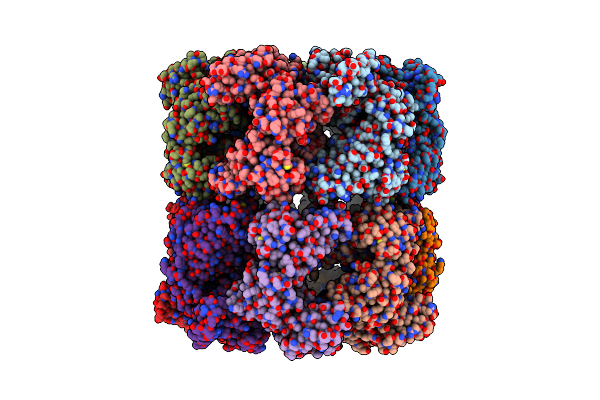 |
Cryo-Em Structure Of Single Empty Ring 2 (Ser2) Of Groel-Ugt1A Complex At 3.2 Ang. Resolution
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
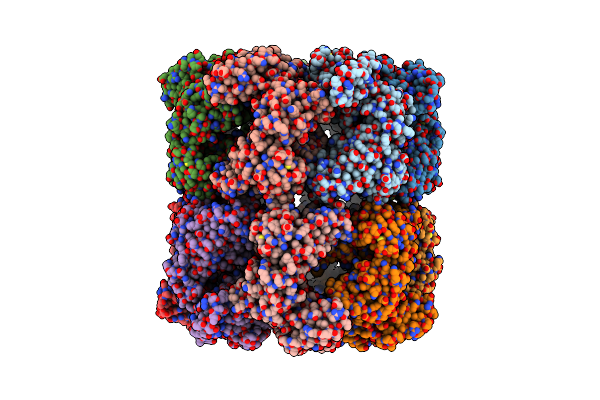 |
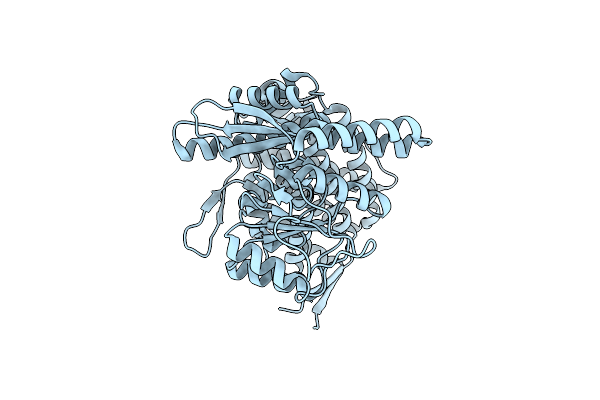
Cryo-Em Structure Of Occupied Ring Subunit 4 (Or4) Of Groel Complexed With Polyalanine Model Of Ugt1A From Groel-Ugt1A Double Occupied Ring Complex
Organism: Escherichia coli, Homo sapiens
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
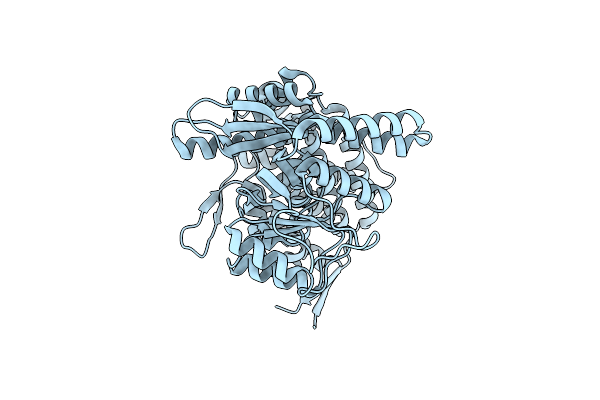
Cryo-Em Structure Of Empty Ring Subunit 1 (Er1) From Single Empty Ring Of Groel-Ugt1A Complex
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
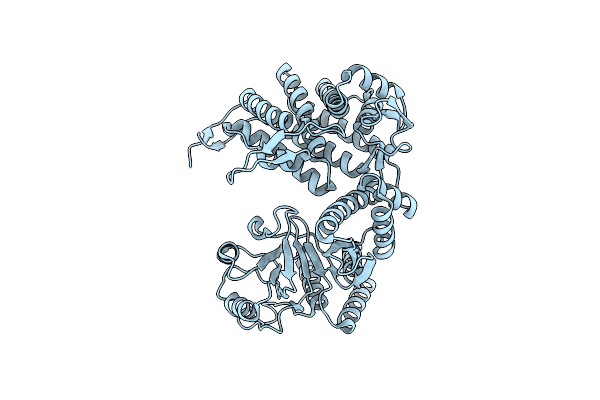
Cryo-Em Structure Of Empty Ring Subunit 2 (Er2) From Groel-Ugt1A Single Empty Ring Complex
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
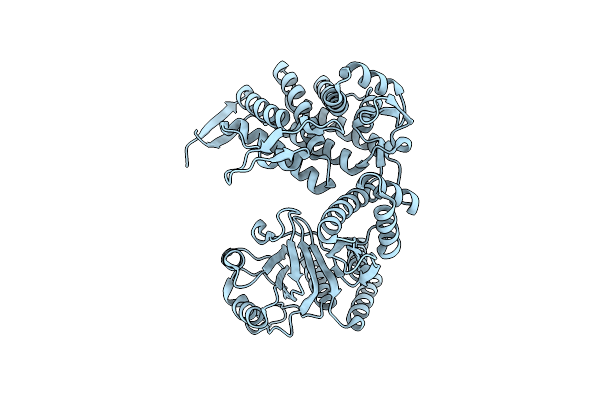
Cryo-Em Structure Of Occupied Ring Subunit 1 (Or1) Of Groel From Groel-Ugt1A Double Occupied Ring Complex
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
Cryo-Em Structure Of Occupied Ring Subunit 2 (Or2) Of Groel From Groel-Ugt1A Double Occupied Ring Complex
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
Cryo-Em Structure Of Occupied Ring Subunit 3 (Or3) Of Groel From Groel-Ugt1A Double Occupied Ring Complex
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
Cryo-Em Structure Of Occupied Ring Subunit 4 (Or4) Of Groel From Groel-Ugt1A Double Occupied Ring Complex
Organism: Escherichia coli
Method: ELECTRON MICROSCOPY Release Date: 2023-05-03 Classification: CHAPERONE |
 |
Time-Resolved Serial Femtosecond Crystallography Reveals Early Structural Changes In Channelrhodopsin: Dark State Structure
Organism: Chlamydomonas reinhardtii
Method: X-RAY DIFFRACTION Resolution:2.30 Å Release Date: 2021-04-07 Classification: MEMBRANE PROTEIN Ligands: RET, OLC |
 |
Time-Resolved Serial Femtosecond Crystallography Reveals Early Structural Changes In Channelrhodopsin: 4 Ms Structure
Organism: Chlamydomonas reinhardtii
Method: X-RAY DIFFRACTION Resolution:2.50 Å Release Date: 2021-04-07 Classification: MEMBRANE PROTEIN Ligands: RET, OLC |
 |
Time-Resolved Serial Femtosecond Crystallography Reveals Early Structural Changes In Channelrhodopsin: 1 Microsecond Structure
Organism: Chlamydomonas reinhardtii
Method: X-RAY DIFFRACTION Resolution:2.50 Å Release Date: 2021-04-07 Classification: MEMBRANE PROTEIN Ligands: RET, OLC |
 |
Time-Resolved Serial Femtosecond Crystallography Reveals Early Structural Changes In Channelrhodopsin: 50 Microsecond Structure
Organism: Chlamydomonas reinhardtii
Method: X-RAY DIFFRACTION Resolution:2.50 Å Release Date: 2021-04-07 Classification: MEMBRANE PROTEIN Ligands: RET, OLC |
 |
Time-Resolved Serial Femtosecond Crystallography Reveals Early Structural Changes In Channelrhodopsin: 250 Microsecond Structure
Organism: Chlamydomonas reinhardtii
Method: X-RAY DIFFRACTION Resolution:2.50 Å Release Date: 2021-04-07 Classification: MEMBRANE PROTEIN Ligands: RET, OLC |
 |
Time-Resolved Serial Femtosecond Crystallography Reveals Early Structural Changes In Channelrhodopsin: 1 Ms Structure
Organism: Chlamydomonas reinhardtii
Method: X-RAY DIFFRACTION Resolution:2.50 Å Release Date: 2021-04-07 Classification: MEMBRANE PROTEIN Ligands: RET, OLC |

