Search Count: 10
All
Selected
 |
Substitution Of Asp193 To Asn At The Active Site Of Ribulose-1,5-Bisphosphate Carboxylase Results In Conformational Changes
Organism: Rhodospirillum rubrum
Method: X-RAY DIFFRACTION Resolution:2.60 Å Release Date: 1994-01-31 Classification: LYASE(CARBON-CARBON) |
 |
Cumulative Site-Directed Charge-Change Replacements In Bacteriophage T4 Lysozyme Suggest That Long-Range Electrostatic Interactions Contribute Little To Protein Stability
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.80 Å Release Date: 1991-10-15 Classification: HYDROLASE (O-GLYCOSYL) |
 |
Cumulative Site-Directed Charge-Change Replacements In Bacteriophage T4 Lysozyme Suggest That Long-Range Electrostatic Interactions Contribute Little To Protein Stability
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.80 Å Release Date: 1991-10-15 Classification: HYDROLASE (O-GLYCOSYL) |
 |
Cumulative Site-Directed Charge-Change Replacements In Bacteriophage T4 Lysozyme Suggest That Long-Range Electrostatic Interactions Contribute Little To Protein Stability
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.70 Å Release Date: 1991-10-15 Classification: HYDROLASE (O-GLYCOSYL) |
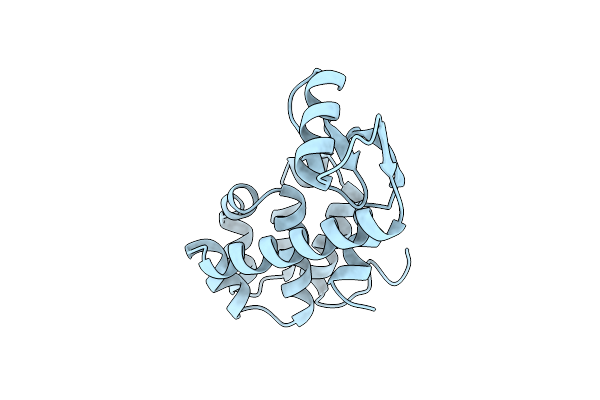 |
Cumulative Site-Directed Charge-Change Replacements In Bacteriophage T4 Lysozyme Suggest That Long-Range Electrostatic Interactions Contribute Little To Protein Stability
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.70 Å Release Date: 1991-10-15 Classification: HYDROLASE (O-GLYCOSYL) |
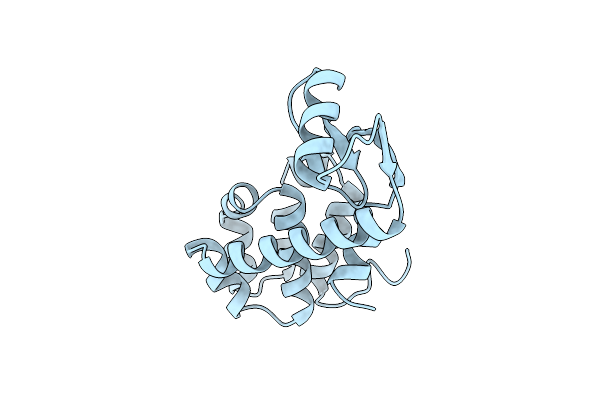 |
Cumulative Site-Directed Charge-Change Replacements In Bacteriophage T4 Lysozyme Suggest That Long-Range Electrostatic Interactions Contribute Little To Protein Stability
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.70 Å Release Date: 1991-10-15 Classification: HYDROLASE (O-GLYCOSYL) |
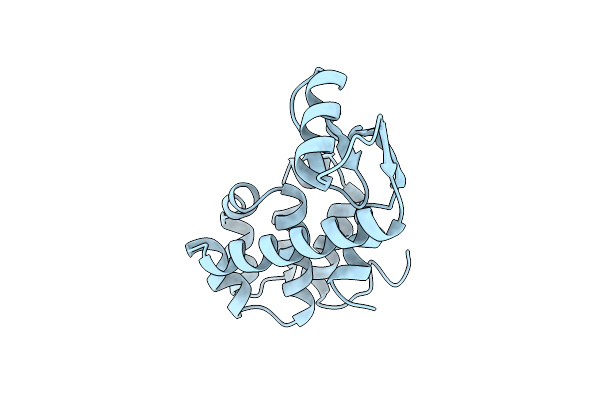 |
Cumulative Site-Directed Charge-Change Replacements In Bacteriophage T4 Lysozyme Suggest That Long-Range Electrostatic Interactions Contribute Little To Protein Stability
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.70 Å Release Date: 1991-10-15 Classification: HYDROLASE (O-GLYCOSYL) |
 |
Contributions Of Left-Handed Helical Residues To The Structure And Stability Of Bacteriophage T4 Lysozyme
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.85 Å Release Date: 1990-01-15 Classification: HYDROLASE (O-GLYCOSYL) |
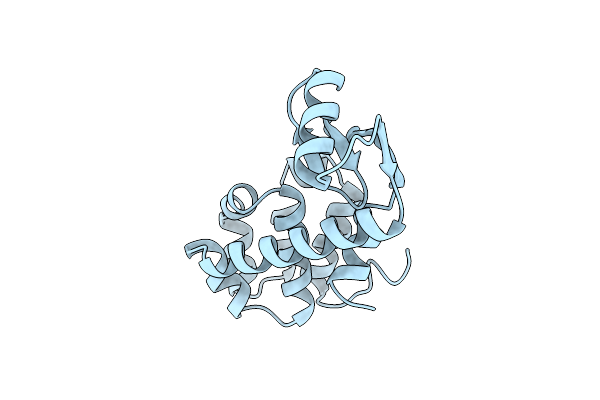 |
Contributions Of Left-Handed Helical Residues To The Structure And Stability Of Bacteriophage T4 Lysozyme
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.70 Å Release Date: 1990-01-15 Classification: HYDROLASE (O-GLYCOSYL) |
 |
Contributions Of Left-Handed Helical Residues To The Structure And Stability Of Bacteriophage T4 Lysozyme
Organism: Enterobacteria phage t4
Method: X-RAY DIFFRACTION Resolution:1.70 Å Release Date: 1990-01-15 Classification: HYDROLASE (O-GLYCOSYL) |

