Search Count: 7
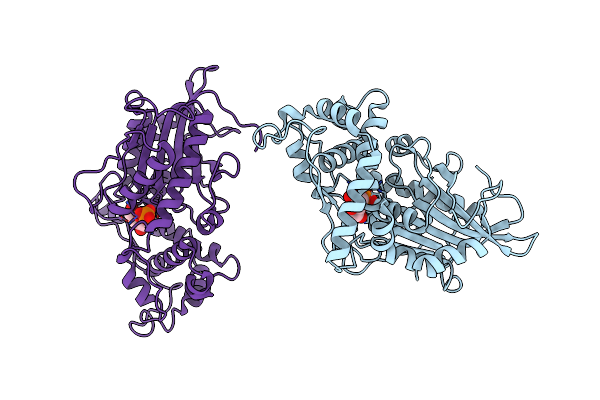 |
Crystal Structure Of Escherichia Coli Periplasmic Glucose-1-Phosphatase H18A Mutant Complexed With Glucose-1-Phosphate
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.40 Å Release Date: 2004-01-13 Classification: HYDROLASE Ligands: XGP |
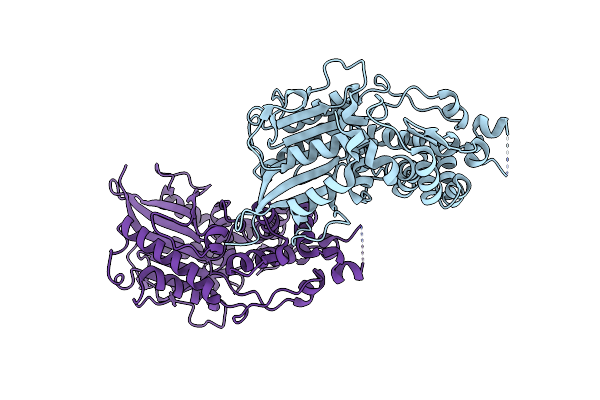 |
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.30 Å Release Date: 2000-08-03 Classification: HYDROLASE |
 |
Crystal Structure Of Escherichia Coli Phytase At Ph 5.0 With Hg2+ Cation Acting As An Intermolecular Bridge
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.40 Å Release Date: 2000-08-03 Classification: HYDROLASE Ligands: HG |
 |
Crystal Structure Of Tungstate Complex Of Escherichia Coli Phytase At Ph 6.6 With Tungstate Bound At The Active Site And With Hg2+ Cation Acting As An Intermolecular Bridge
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.38 Å Release Date: 2000-08-03 Classification: HYDROLASE Ligands: HG, WO4 |
 |
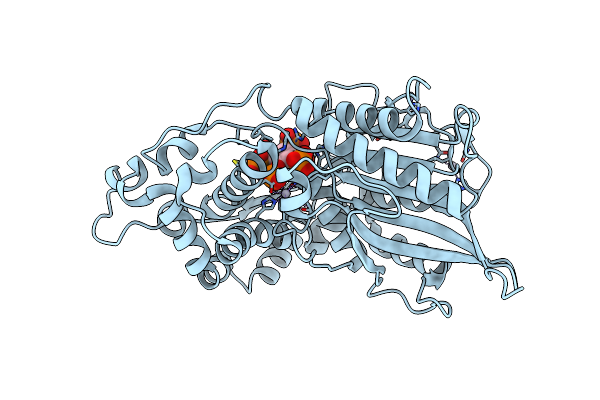
Crystal Structure Of Phytate Complex Of Escherichia Coli Phytase At Ph 6.6. Phytate Is Bound With Its 3-Phosphate In The Active Site. Hg2+ Cation Acts As An Intermolecular Bridge
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.28 Å Release Date: 2000-08-03 Classification: HYDROLASE Ligands: HG, IHP |
 |
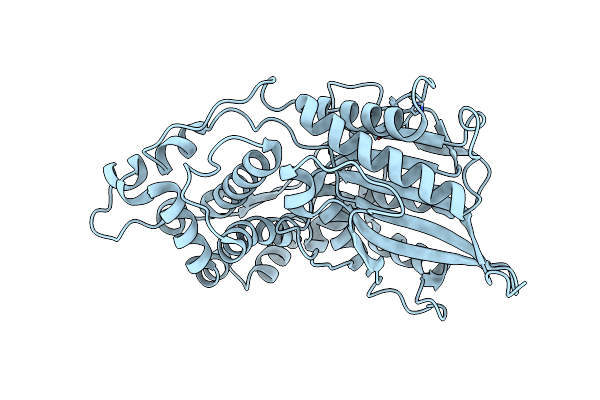
Crystal Structure Of Phytate Complex Escherichia Coli Phytase At Ph 5.0. Phytate Is Bound With Its 3-Phosphate In The Active Site. Hg2+ Cation Acts As An Intermolecular Bridge
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.05 Å Release Date: 2000-08-03 Classification: HYDROLASE Ligands: HG, IHP |
 |
Crystal Structure Of Escherichia Coli Phytase At Ph 6.6 With Hg2+ Cation Acting As An Intermolecular Bridge
Organism: Escherichia coli
Method: X-RAY DIFFRACTION Resolution:2.25 Å Release Date: 2000-08-02 Classification: HYDROLASE Ligands: HG |

