Deposition Date
2014-12-18
Release Date
2015-06-10
Last Version Date
2024-01-10
Entry Detail
PDB ID:
4XCO
Keywords:
Title:
Signal-sequence induced conformational changes in the signal recognition particle
Biological Source:
Source Organism:
Methanocaldococcus jannaschii (Taxon ID: 2190)
Host Organism:
Method Details:
Experimental Method:
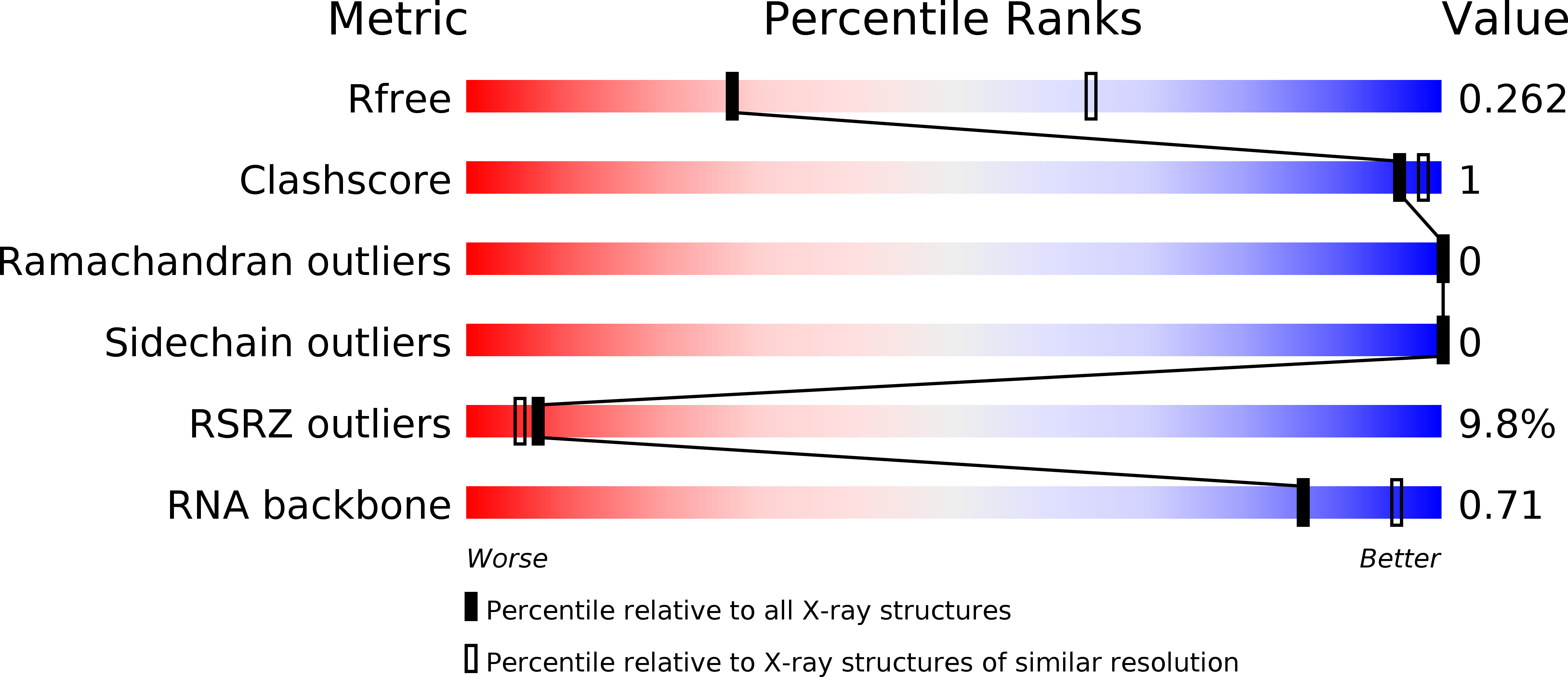
Resolution:
2.90 Å
R-Value Free:
0.25
R-Value Work:
0.20
R-Value Observed:
0.21
Space Group:
P 2 21 21