Deposition Date
2013-07-30
Release Date
2013-12-04
Last Version Date
2024-02-28
Entry Detail
PDB ID:
4LY6
Keywords:
Title:
Nucleotide-induced asymmetry within ATPase activator ring drives s54-RNAP interaction and ATP hydrolysis
Biological Source:
Source Organism:
Aquifex aeolicus (Taxon ID: 63363)
Host Organism:
Method Details:
Experimental Method:
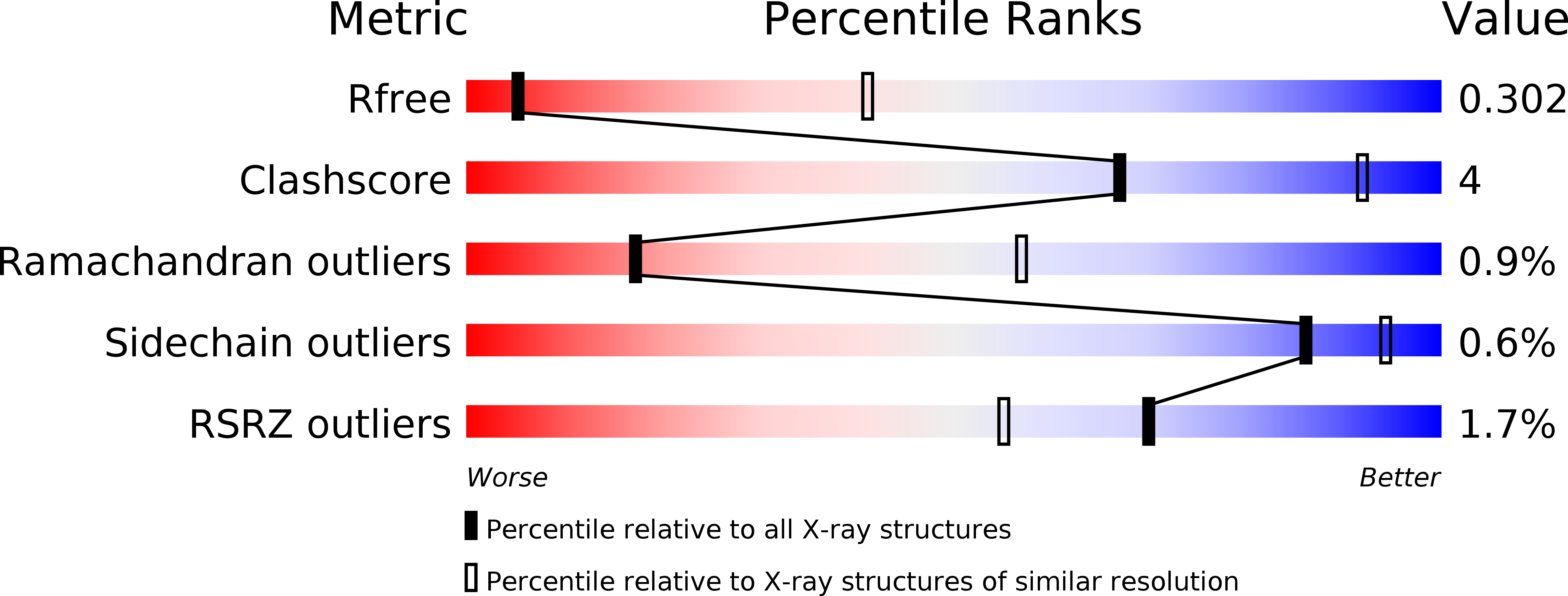
Resolution:
3.60 Å
R-Value Free:
0.30
R-Value Work:
0.25
R-Value Observed:
0.26
Space Group:
P 1