Deposition Date
2013-08-02
Release Date
2014-02-19
Last Version Date
2024-05-08
Entry Detail
PDB ID:
4C0H
Keywords:
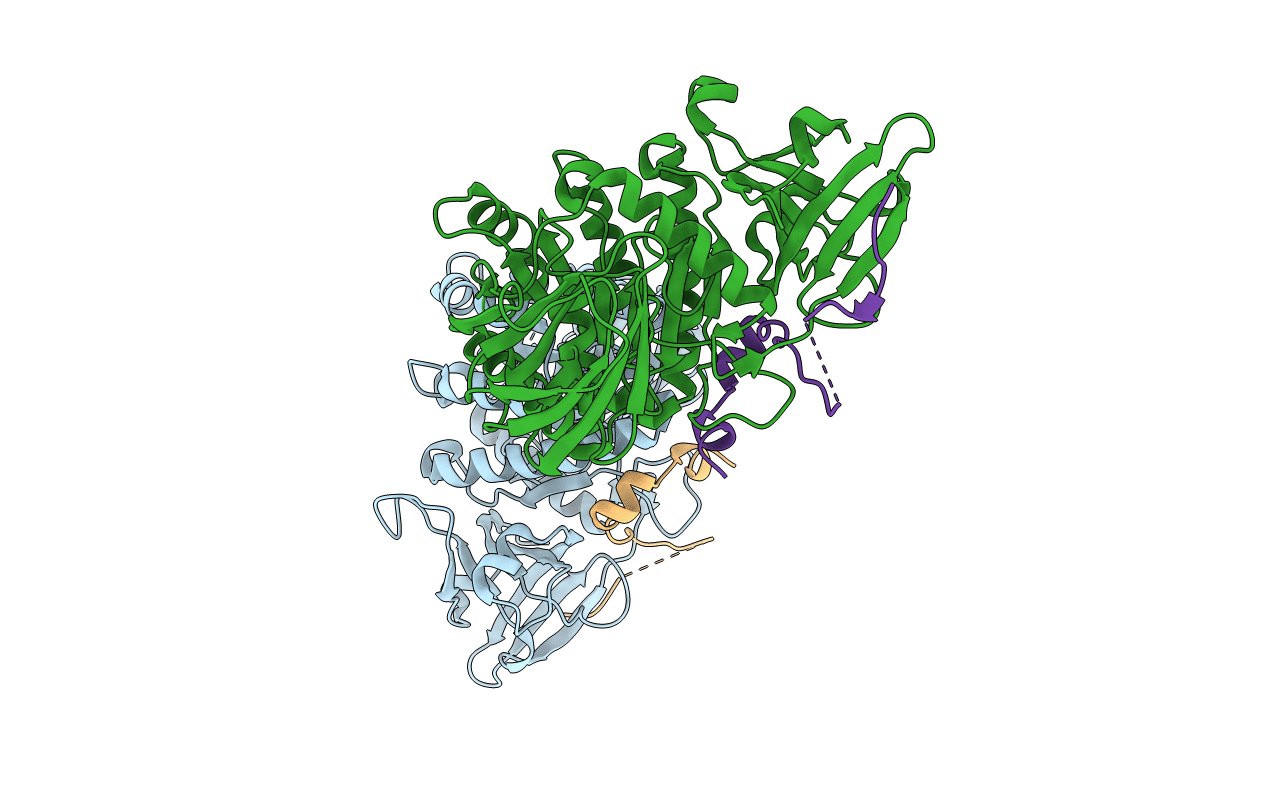
Title:
Extended interface between Pcf11p and Clp1p and structural basis for ATP loss in Gly135Arg point mutant
Biological Source:
Source Organism:
SACCHAROMYCES CEREVISIAE (Taxon ID: 4932)
Host Organism:
Method Details:
Experimental Method:
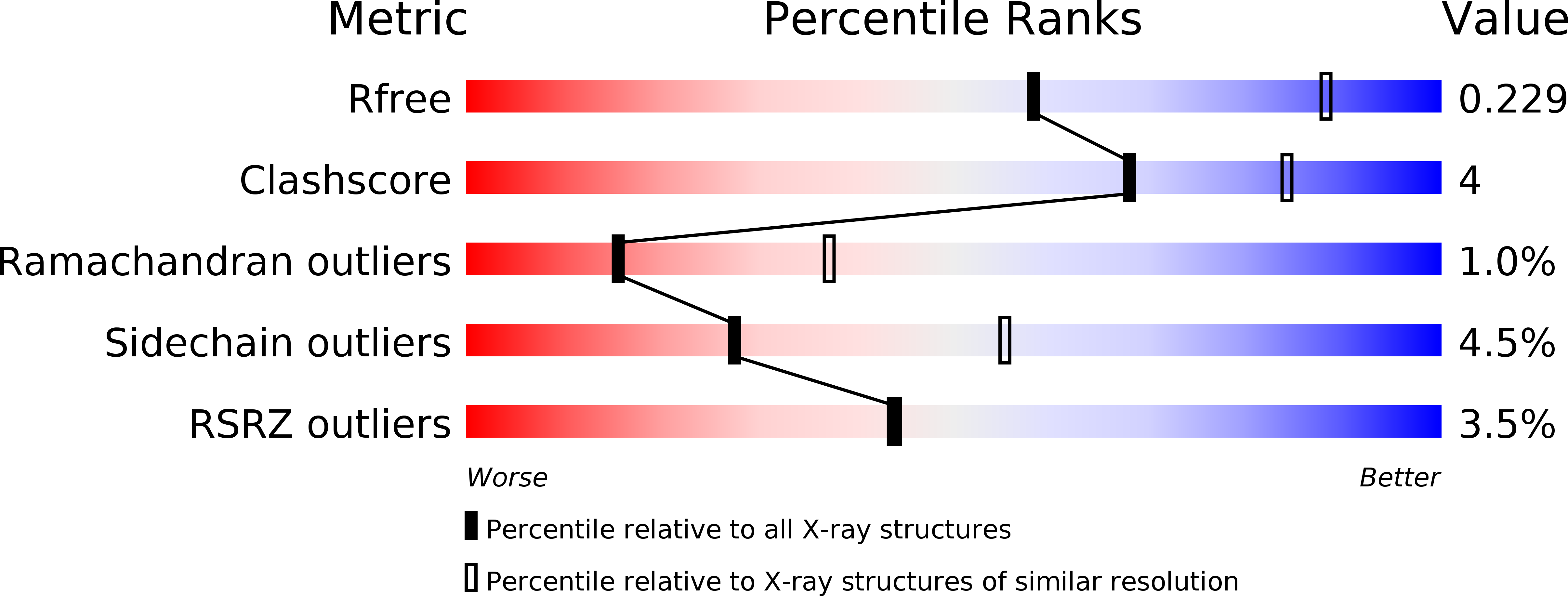
Resolution:
2.70 Å
R-Value Free:
0.22
R-Value Work:
0.19
R-Value Observed:
0.19
Space Group:
P 21 21 21