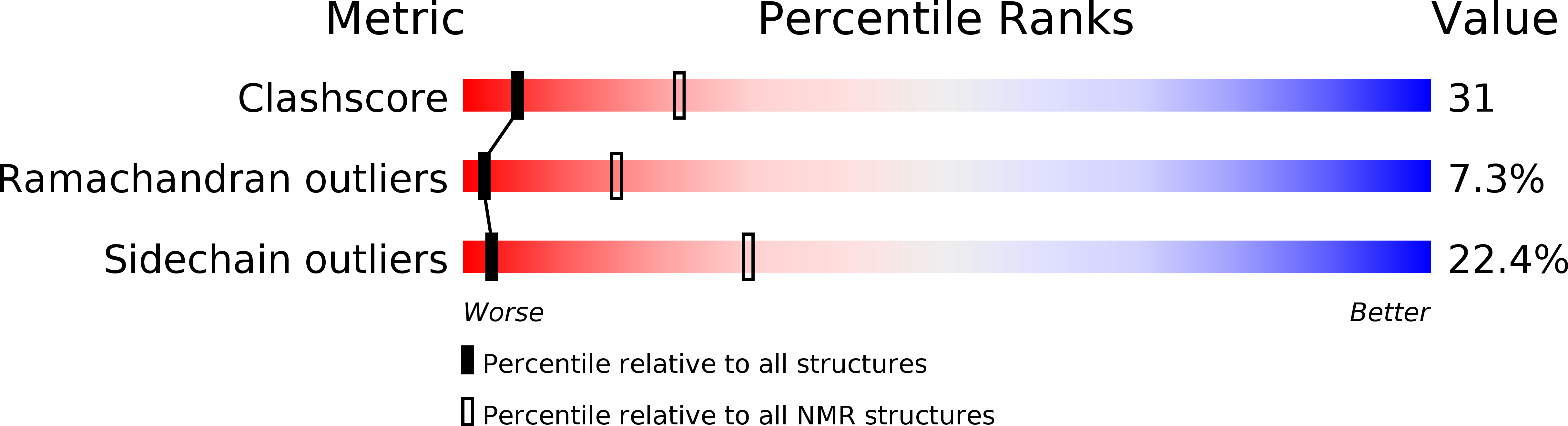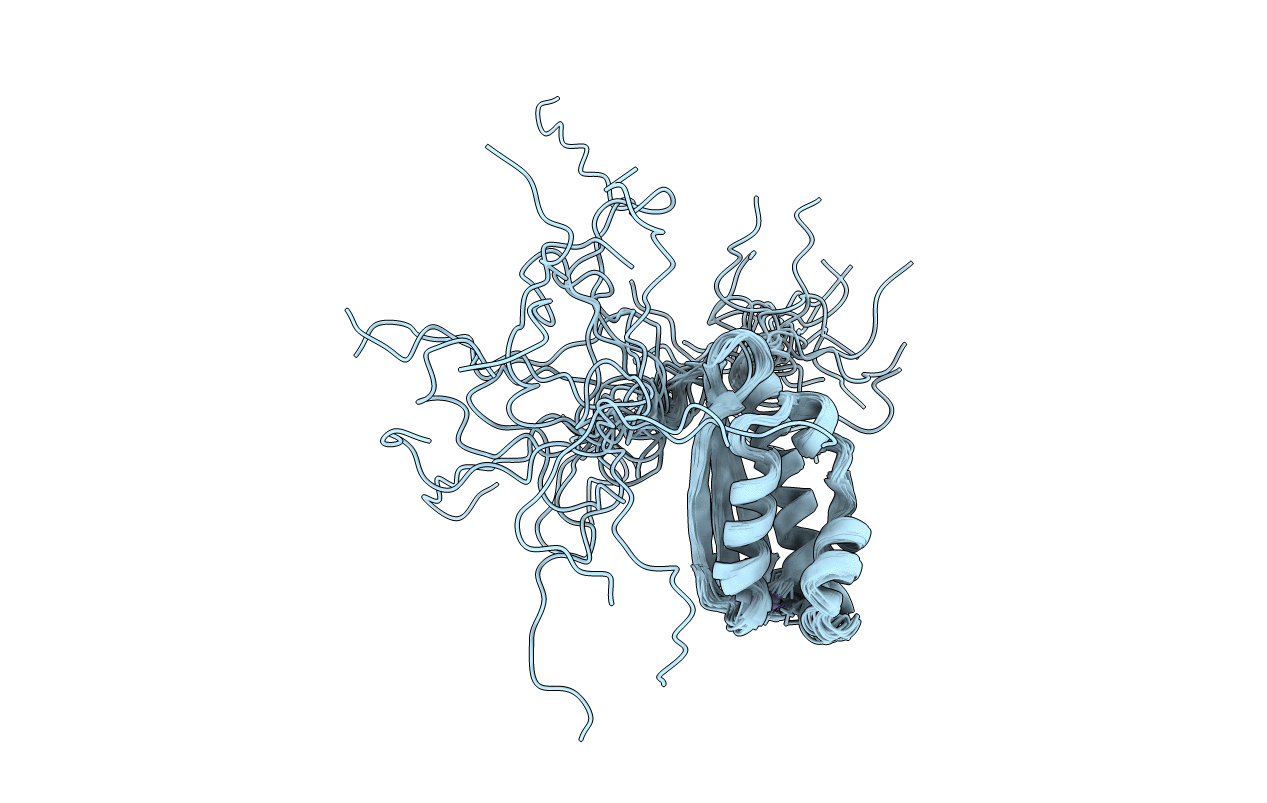
Deposition Date
2003-10-30
Release Date
2004-11-09
Last Version Date
2024-05-01
Entry Detail
PDB ID:
1R9P
Keywords:
Title:
Solution NMR Structure Of The Haemophilus Influenzae Iron-Sulfur Cluster Assembly Protein U (IscU) with Zinc Bound at the Active Site. Northeast Structural Genomics Consortium Target IR24.
Biological Source:
Source Organism:
Haemophilus influenzae (Taxon ID: 727)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
25
Conformers Submitted:
20
Selection Criteria:
structures with the lowest energy