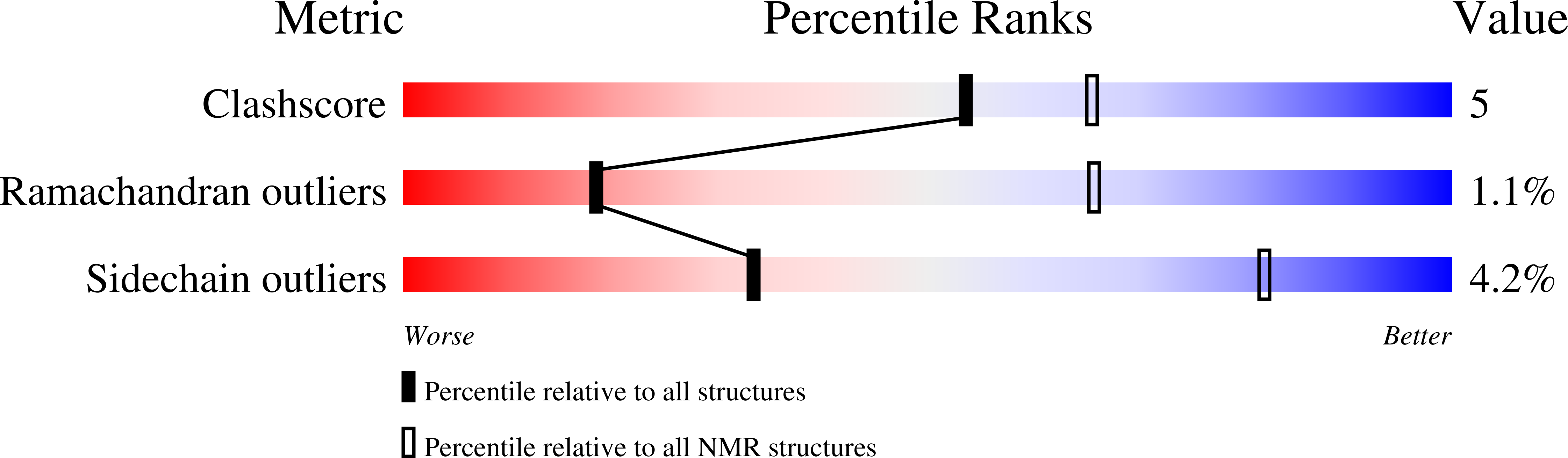Deposition Date
2003-12-04
Release Date
2004-12-14
Last Version Date
2024-05-22
Entry Detail
PDB ID:
1RQ6
Keywords:
Title:
Solution structure of ribosomal protein S17E from Methanobacterium Thermoautotrophicum, Northeast Structural Genomics Consortium Target TT802 / Ontario Center for Structural Proteomics Target Mth0803
Biological Source:
Source Organism:
Methanothermobacter thermautotrophicus (Taxon ID: 145262)
Host Organism:
Method Details:
Experimental Method:
Conformers Calculated:
112
Conformers Submitted:
15
Selection Criteria:
structures with the least restraint violations,target function